Revolutionary catalyst discovered
Metal-organic-framework catalyst provides new tool for industry
Researchers have developed a new catalyst material that outperforms benchmarks and opens the door to significant advances in petroleum refinement and industrial applications. It’s an industry first, and there’s plenty of room to build on their new discovery.
Catalysts speed up chemical reactions without themselves being used up. Catalysis is a $16-billion industry, which contributes up to 35 per cent of the global Gross Domestic Product.
“We don’t see it, but we rely on catalysis an awful lot. If we could develop a better catalyst, that could have effects in all kinds of areas of our lives,” says Dr. Shengqian Ma.
The space within nanoporous materials provides virtually unlimited room for imagination
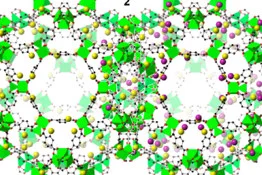
Ma’s University of South Florida research group has done just that, having developed a Metal-Organic Framework (MOF), which outperforms zeolite materials in catalysis on several counts. While you may not have heard of them, zeolites play a major role in a range of catalytic applications, most prominently in gas and petroleum refining. Ma’s MOF material combines an organic Brønsted acid framework with an aluminum Lewis acid center to create a more efficient catalysis.
“There’s synergy here. It’s this idea of one plus one is larger than two. The combined acids are much better than the zeolite and much better than on their own,” says Ma.
The MOF’s performance is impressive. In their experiments, the Ma group compared an identical sample reaction with standard zeolites and the new MOF material. For one thing, zeolites start to lose their efficiency after an hour, and are generally completely useless after five hours. The MOF not only kept working after six hours, but it actually increased in efficiency, peaking at more than 99 per cent yields after two hours.
Efficiency is a very important criterion for the catalyst, and it is related to how complete the reactants can be converted and how much by-products will be generated.
The exceptionally high efficiency observed in the MOF catalyst is mainly because of the mutual promotion between the organic Brønsted acid site and the aluminum Lewis acid center when combined within the MOF. The coordination environment of the aluminum in the MOF catalyst was studied using the spectroscopy capabilities of the Canadian Light Source, providing key insight into the interactions between the two acids.

In addition, the pores which allow molecules to enter and exit MOFs during the catalysis process are much larger than those of zeolite materials. Larger pores prevent the reaction from stalling or overheating, which likely helps give MOFs their long effective life. In principle, MOFs could combine inorganic chemistry and organic chemistry and they feature designability, tunability, and modularity, which will likely be usable in a wider range of applications than zeolites, and with more flexibility.
At the Ma lab, there is a palpable excitement.
“The space within nanoporous materials provides virtually unlimited room for imagination,” says Ma. “This is a huge step forward in the field. We can see now how MOFs can be really useful and build on that.”