This post first appeared on the Canadian Light Source.
Researchers from McGill University and Université du Québec à Montreal (UQAM) have found a new approach to making inexpensive batteries that can not only hold large amounts of charge but also recharge quickly.
Their work focuses on improving lithium ion batteries, rechargeable cells that are used in electric vehicles, power tools, phones and more.
“The work that we’ve done at the CLS is going to open up the door to be able to make batteries that can be charged faster, which will be one of the ways that we can start implementing them in real use cases as soon as possible,” says McGill researcher Jeremy Dawkins, the lead author of a recent paper on the work published in the journal ChemElectroChem.
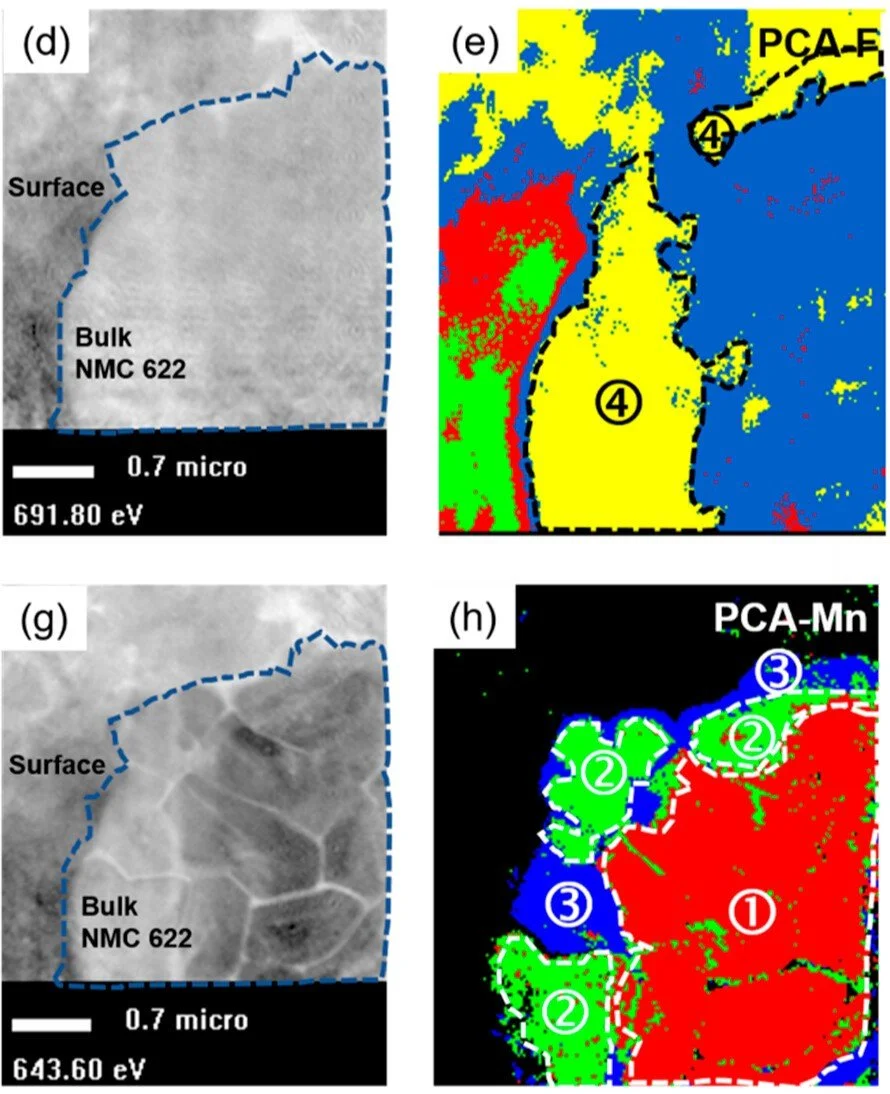
To understand how a battery performs, researchers need to see what’s going on inside while it is being used. This is challenging to do in most labs, but the Canadian Light Source (CLS) synchrotron at the University of Saskatchewan (USask) offers the bright, intense x-ray light required to peer into a working battery.
Lithium ion batteries can be made of a combination of different materials, which researchers tweak to get the performance they want.
“We want to combine two materials and use the benefits of both of them. So we want to have one material that is capable of fast charging and the other one that is capable of having a huge capacity,” says Bastian Krueger, a UQAM battery researcher.
The researchers produced a battery by mixing a known fast-charging material with a high-capacity one, and experimented with different ways to combine them. The CLS enabled them to image the lithium ions – which act as a bottleneck for fast charging – so that they could monitor the battery chemistry while it was being charged. The team found that a layered, sandwich-like approach worked best.
“What’s particularly interesting about the layered battery we used in this work is that the lithium ion is able to move more efficiently through the cell, which opens up the possibilities to do stuff like fast charging, which is important if you want to, for example, fast charge your cell phone in 5 minutes or charge your car in 10 minutes,” says Dawkins.
Dawkins, Jeremy IG, Yani Pan, Mohammadreza Z. Ghavidel, Johann Geissler, Bastian Krueger, Danny Chhin, Hui Yuan et al. "Exploring the Synergistic Effects of Dual‐Layer Electrodes for High Power Li‐Ion Batteries." ChemElectroChem (2023): e202300279. https://doi.org/10.1002/celc.202300279